It’s been five years since the first successful clinical trial results showed that the AstraZeneca/Oxford COVID-19 vaccine was effective (November 2020). The vaccine went on to prevent an estimated six million deaths. Since then, the Pandemic Sciences Institute has been established in Oxford as a multi-disciplinary, University-wide initiative to build upon the model of innovation, collaboration and agility that yielded such critical breakthroughs.
Oxfordshire has several globally-renowned centres of vaccine expertise. The Jenner Institute develops vaccines and carries out clinical trials for diseases including malaria, tuberculosis, and Ebola. In 2023, the WHO recommended the global roll-out of the R21/Matrix-M™ malaria vaccine – the culmination of 30 years of research at the Jenner. The vaccine has been licensed for use in Ghana, Nigeria and Burkina Faso, and Ivory Coast was the first country to roll out the vaccine.
The Draper Lab at the University of Oxford focuses on the development of novel and improved approaches to blood-stage malaria vaccine design. A global consortium, co-led by the Draper Lab’s Professor Angela Minassian in Oxford and Associate Professor H. Magloire Natama in Burkina Faso, has recently been established to advance the development of a next-generation malaria vaccine that will target multiple stages of the parasite’s lifecycle. The consortium will combine R21/Matrix-M™ with optimising protection against the blood-stage of infection to deliver the most comprehensive protection yet for young children.
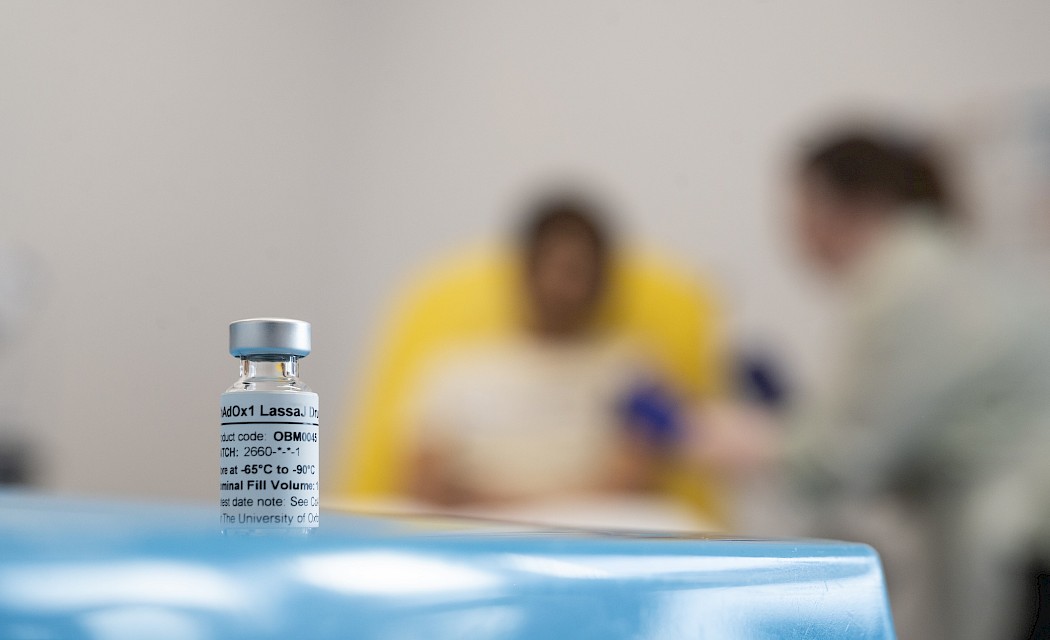
Other Oxford vaccine breakthroughs this year include the first-in-human trial of a Lassa vaccine, developed by researchers at the the Pandemic Sciences Institute, marking a major milestone in the fight against the deadly Lassa fever virus, a WHO priority pathogen that poses a significant public health risk. The trial, conducted by the Oxford Vaccine Group, and funded by the Coalition for Epidemic Preparedness Innovations (CEPI), will assess the safety and immune response of the ChAdOx1 Lassa vaccine. 31 people aged 18-55 will participate in the trial in total.
The Oxford Vaccine Group is also leading a Phase 1 clinical trial of a vaccine to protect people from deadly Nipah virus. This has been granted support from the PRIority MEdicines (PRIME) scheme offered by Europe’s medicines regulator, the European Medicines Agency (EMA). Oxford is the first UK academic institution to be awarded this designation. PRIME provides targeted scientific and regulatory support to medications designed to address conditions with an unmet medical need; there are currently no licensed vaccines or treatments for Nipah virus, a deadly recognised by the World Health Organization as a research priority due to its pandemic potential. A vaccine is urgently needed as the disease can be fatal in up to 85% of cases.
These examples show how Oxfordshire’s world-class innovation has far-reaching impact, benefiting the health of people all around the world, while creating opportunity and driving sustainable growth.
Image credit: Courtesy of University of Oxford, photos by John Cairns